
Blog
Pain Relief Tablets Import Guide
Navigating the complex world of pain relief tablet importation requires understanding multiple federal agencies, state programs, and international regulations. With prescription drug prices averaging 44% higher in the United States compared to countries like Canada, many individuals and organizations are exploring legal importation options. However, the regulatory landscape involves the FDA, drug enforcement administration, and various state-level programs, each with specific requirements for different types of medications. This pain relief tablets import guide aims to clarify these complexities.
This comprehensive guide explains the legal pathways for importing pain relief tablets, from personal use quantities for individual patients to large-scale state importation programs. Whether you’re a healthcare professional helping patients access affordable medications, a traveler concerned about carrying prescribed medicines internationally, or someone exploring commercial importation opportunities, understanding these regulations is essential for compliance and safety.
Utilizing this pain relief tablets import guide will enhance your knowledge about the various aspects of pain relief tablets import guide.

Understanding Pain Relief Tablet Importation
This pain relief tablets import guide will help you navigate the necessary steps and regulations for pain relief tablets import guide.
Pain relief tablets containing controlled substances require special FDA and DEA approval for importation due to their potential for abuse and diversion. The regulatory framework distinguishes between over the counter medications like acetaminophen and ibuprofen, which face fewer restrictions, and prescription drug products containing opioids or other controlled substances that trigger additional oversight.
In addition, this pain relief tablets import guide will provide insights into pain relief tablets import guide.
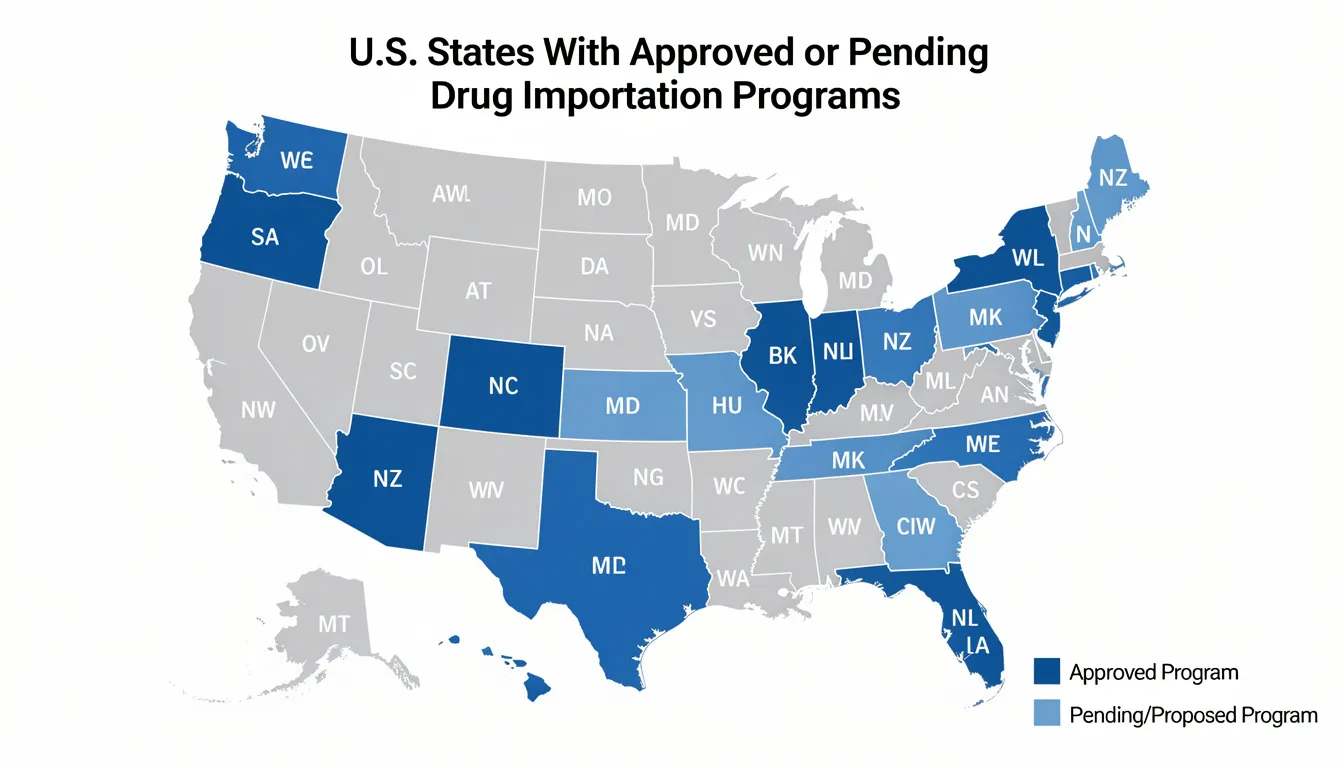
Florida became the first state authorized in January 2024 to import prescription drugs from Canada under FDA’s Section 804 Importation Program, marking a significant milestone in drug importation policy. This program targets specific medications including pain management drugs for conditions like HIV/AIDS and cancer, demonstrating how state-level initiatives can address medication access challenges while maintaining safety standards. Following this pain relief tablets import guide can assist in understanding these ongoing developments.
Following this pain relief tablets import guide will ensure compliance in your pain relief tablets import guide processes.
Personal importation is limited to 90-day supplies with physician documentation and specific conditions that meet FDA criteria for serious medical conditions. The regulations require that effective treatment must be unavailable through commercial or clinical means in the United States, and individuals must provide evidence that the medication is for their own use without known commercialization to other persons residing in the U.S.
Consult this pain relief tablets import guide before making decisions regarding your pain relief tablets import guide.
Commercial importation requires extensive licensing, quality testing, and compliance with FDA regulations including Good Manufacturing Practices and establishment licensing for foreign manufacturing sites. Companies seeking to import drugs must demonstrate supply chain security and meet the same safety standards applied to domestic pharmaceutical products.
Many common pain relievers like opioids, codeine, and certain NSAIDs face strict importation restrictions due to their classification as controlled substances. These medications require coordination between the FDA and DEA, with the drug enforcement administration making final determinations about importation eligibility for personal use quantities.
This is crucial information that the pain relief tablets import guide should cover.
Legal Framework for Pain Relief Tablet Imports
The 2000 Medicine Equity and Drug Safety (MEDS) Act allows pharmacists to import drugs from certain countries if certified safe, establishing the foundational framework for prescription drug importation while requiring HHS Secretary certification that such programs pose no additional risk to public health and safety.
Adhering to the pain relief tablets import guide is vital for successful importation.
The 2003 Medicare Modernization Act restricts importation to drugs from Canada with HHS regulation, limiting the geographic scope of legal importation programs and establishing Canada as the primary source country for state-level importation initiatives under most circumstances.
The 2020 Section 804 Importation Program creates a pathway for states to import prescription drugs from Canada, representing the most significant expansion of importation opportunities in decades. This program requires states to submit detailed applications demonstrating safety measures, supply chain security, and projected cost savings for their residents.
The FDA oversees pharmaceutical importation policies under the food and drug and Cosmetic Act, maintaining authority over which drug products can enter the United States and under what conditions. The agency’s regulatory framework emphasizes that imported medications must meet identical safety and efficacy standards as domestically manufactured products.
Understanding the implications of the pain relief tablets import guide will help you avoid potential pitfalls.
The Drug Enforcement Administration regulates controlled substances under the Controlled Substances Act, creating an additional layer of oversight for pain relief tablets containing opioids or other controlled ingredients. This dual regulatory structure requires coordination between agencies when evaluating importation requests for medications that fall under both FDA and DEA jurisdiction.
Personal Importation Guidelines
Referencing the pain relief tablets import guide will streamline the process.
Personal importation is limited to FDA-approved drugs for serious conditions with no effective treatment available in the United States, ensuring that individuals can access medically necessary medications while preventing abuse of importation privileges for commercial purposes.
The maximum 90-day supply allowed with written confirmation of personal use reflects a balance between patient access and regulatory control. This three month supply limitation helps prevent bulk importation that might indicate commercial intent while providing sufficient medication for individual patient needs.
A thorough review of the pain relief tablets import guide can clarify many uncertainties.
A physician letter is required listing medications, dosages, and medical indications, establishing medical necessity and professional oversight for imported pain relief tablets. The healthcare professional must be doctor licensed in the United States and should document that the treatment begun with the imported medication addresses a serious condition.
Original labeled containers are mandatory to avoid pill organizers during customs inspection, as proper labeling helps customs officials verify medication authenticity and prevents confusion about drug identity. Travelers should maintain medications in their original pharmacy containers with clear identification of the drug manufacturer, active ingredient, and prescribing information.
Keeping the pain relief tablets import guide in mind helps navigate complex regulations.
Personal importation cannot involve commercialization or resale in the United States, distinguishing legitimate personal use from commercial distribution activities. The consumer affirms in writing that imported medications are for personal use and will not be redistributed to other individuals or sold commercially.
Controlled Substance Pain Medications
Strict adherence to the pain relief tablets import guide can prevent legal issues.
Opioids like oxycodone, morphine, and fentanyl require DEA approval for importation due to their high abuse potential and strict federal controls. The drug enforcement administration evaluates each request based on medical necessity, quantity requested, and the individual’s compliance history with controlled substance regulations.
Evaluating each medication against the pain relief tablets import guide is crucial.
Individuals should contact the DEA Office of Diversion Control at (202) 305-8800 for controlled substance permits before attempting to import pain medications containing these ingredients. The agency provides specific guidance about documentation requirements and approval procedures for legitimate medical use.
This pain relief tablets import guide will assist in understanding these regulations.
Valid U.S. prescriptions do not guarantee legal importation in all countries, as destination nations maintain their own drug control laws that may prohibit medications legal in the United States. Travelers must research local laws in addition to U.S. importation requirements to ensure compliance throughout their journey.
Japan prohibits many U.S.-legal pain medications including those containing marijuana derivatives and amphetamines, requiring special import permissions called yakkan shoumei for certain medications. The country’s strict drug laws can result in arrest and prosecution even for medications legally prescribed by U.S. physicians.
Always reference the pain relief tablets import guide before traveling.
The risk of arrest exists even with valid prescriptions when travelling internationally, particularly in countries with zero-tolerance drug policies. Travelers should contact embassy officials in their destination country to verify medication legality and obtain required documentation before departure.

Commercial Importation Requirements
The pain relief tablets import guide will offer additional insights for commercial importers.
Importers must hold Medical Device Establishment License (MDEL) or Drug Establishment License depending on the type of products being imported, ensuring that commercial entities have appropriate regulatory oversight and quality control systems in place.
Foreign manufacturing sites require FDA inspection and Establishment License approval before their products can be imported into the United States. This requirement ensures that overseas facilities meet the same manufacturing standards applied to domestic pharmaceutical companies.
This information is essential to the pain relief tablets import guide.
An 8-digit Drug Identification Number (DIN) is required before marketing imported pain medications, providing unique identification for each product formulation and enabling tracking throughout the distribution chain. Health canada assigns these numbers for products approved in the Canadian market.
Additionally, the pain relief tablets import guide highlights the importance of documentation.
Good Manufacturing Practices (GMP) compliance is mandatory for all imported pharmaceutical products, ensuring consistent quality and safety standards regardless of manufacturing location. Companies must demonstrate adherence to current GMP guidelines through documentation and facility inspections.
Adhering to the pain relief tablets import guide ensures consistent quality.
Letter of Authorization (LOA) and proper labeling are required for investigational products, allowing research institutions and healthcare facilities to import experimental pain relief formulations for clinical trials or compassionate use programs under FDA oversight.
Documentation Requirements
Documentation in line with the pain relief tablets import guide will expedite the process.
Commercial importers must provide product license numbers, establishment license numbers, and Health Canada import documents for each medication shipment. This documentation enables FDA officials to verify product authenticity and regulatory status in both countries.
A Certificate of Pharmaceutical Product (CPP) facilitates international export by providing official verification that medications meet regulatory standards in their country of origin. This document is particularly important for pain relief tablets that may face scrutiny due to their controlled substance content.
Pre-import requests must be submitted to the FDA for each drug batch before importation, allowing agency review of specific shipments and enabling rapid response to quality or safety concerns. This requirement applies even to previously approved products to ensure ongoing compliance.
Relying on the pain relief tablets import guide guarantees thorough compliance.
Quality testing certificates and supply chain security documentation must accompany each shipment to verify product integrity and prevent counterfeit medications from entering the U.S. market. Testing must cover active pharmaceutical ingredients and finished product specifications.
FDA Form 2877 for drug registration and listing of pain relief formulations provides official notification to the agency about imported products and their intended distribution. This form creates a regulatory record linking imported medications to responsible parties in the United States.
State Importation Programs
Florida’s approved program targets 14 drugs including pain management medications for HIV/AIDS and cancer patients, demonstrating how state initiatives can focus on high-impact therapeutic areas where cost savings provide the greatest benefit to vulnerable populations.
State programs will benefit from the pain relief tablets import guide.
Colorado, Vermont, Maine, New Mexico, New Hampshire, North Dakota, and Texas have passed importation laws authorizing their health departments to develop importation programs. These states are in various stages of program development and FDA approval processes.
State programs are limited to Medicaid recipients and state agency clients rather than the general public, ensuring that imported medications primarily benefit individuals with limited financial resources and existing state oversight mechanisms.
Florida’s program projects estimated $183 million savings for the state’s Medicaid program in the first year of operation, illustrating the potential economic impact of well-designed importation initiatives on state healthcare budgets.
The pain relief tablets import guide highlights the potential for significant savings.
States must demonstrate safety and cost savings before FDA approval, requiring comprehensive documentation of quality control measures, supply chain security, and projected financial benefits. The approval process involves extensive review of state capabilities and regulatory compliance systems.
International Travel Considerations
Travelers must understand the pain relief tablets import guide to avoid complications.
Many countries restrict common pain medications legal in the United States, creating potential legal complications for travelers carrying prescribed medicines across international borders. These restrictions vary significantly by destination and can change without notice.
Pseudoephedrine, codeine, and tramadol face restrictions in various international destinations due to their potential for abuse or use in illegal drug manufacturing. Countries may limit quantities, require special permits, or prohibit these substances entirely.
Travelers should contact the destination country’s embassy for specific medication restrictions before travel to obtain current information about prohibited substances and required documentation. Embassy websites often provide detailed guidance about medical exceptions and permit procedures.
The International Narcotics Control Board (INCB) website details country-specific drug restrictions, offering a comprehensive resource for travelers carrying multiple medications or traveling to multiple countries during their trip.
Carrying medications in original containers with pharmacy labels during international travel helps customs officials verify prescription legitimacy and reduces the risk of detention or confiscation. Labels should clearly identify the prescribing physician, patient name, and medication details.
High-Risk Destinations
Japan prohibits many over-the-counter pain relievers including allergy medications containing stimulants like pseudoephedrine, requiring travelers to obtain yakkan shoumei import permission from the Ministry of Health before arrival.
Middle Eastern countries strictly regulate opioid pain medications and sedatives, with some nations imposing severe penalties including imprisonment for possession of controlled substances without proper authorization. The risk related to carrying such medications is significant.
The European Union requires additional documentation for controlled substance pain relievers, though individual member countries may have varying requirements. Travelers should verify requirements for each country they plan to visit.
Contact Japan’s Ministry of Health for yakkan shoumei import permission before travel if carrying any questionable medications. The application process requires medical documentation and can take several weeks to complete.
U.S. Embassy websites provide country-specific medication warnings and restrictions, offering the most current information about enforcement practices and legal requirements for American travelers.
Safety and Compliance Requirements
Imported pain tablets must meet the same FDA standards as domestic products, ensuring that cost savings from importation do not compromise patient safety or medication effectiveness. All imported drugs undergo the same rigorous quality testing and approval processes.
Quality testing is required for active pharmaceutical ingredients and finished products to verify potency, purity, and stability throughout the importation and distribution process. Testing must be conducted by qualifying laboratories with appropriate certification and oversight.
Canadian Health Products and Food Branch approval is required for eligible medications under the Section 804 Importation Program, ensuring that imported drugs meet Canadian regulatory standards in addition to U.S. requirements.
Risk Evaluation and Mitigation Strategies (REMS) drugs are excluded from importation programs due to their classification as high-risk products requiring specialized monitoring and distribution controls that cannot be maintained through importation channels.
Compliance with the pain relief tablets import guide is mandatory for safety.
Non-compliant products are subject to detention, refusal, seizure, or destruction by FDA officials at ports of entry. The agency maintains authority to prevent entry of any products that fail to meet safety standards or lack proper documentation.
Cost Considerations and Savings
Canadian prescription drug prices average 44% of U.S. prices due to government cost controls and negotiated pricing mechanisms that limit pharmaceutical company pricing flexibility in the Canadian market.
Personal importation may reduce individual medication costs but involves legal and safety risks that patients must weigh against potential savings. The complexity of regulations and documentation requirements can also create practical barriers to access.
State programs focus on institutional savings rather than individual consumer access, prioritizing Medicaid populations and state employees to maximize public health benefits and ensure appropriate oversight of imported medications.
Shipping, testing, and compliance costs may offset potential savings from importation, particularly for smaller quantities or specialized medications requiring extensive quality verification and regulatory compliance measures.
This landscape emphasizes the importance of the pain relief tablets import guide.
The Canadian government warns that increased U.S. importation could cause drug shortages and impact medication access for Canadian patients, creating diplomatic and practical challenges for expanded importation programs.
This comprehensive pain relief tablets import guide demonstrates the complexity of current regulations and the multiple pathways available for legal importation. Whether pursuing personal importation for medical conditions, exploring state program eligibility, or planning international travel with prescribed medicines, understanding these regulatory requirements is essential for legal compliance and patient safety. The evolving landscape of importation policies continues to create new opportunities while maintaining strict safety standards to protect public health. This pain relief tablets import guide will be your go-to resource.
